
With a view to coordinating and standardizing the administration of medical devices in European countries,
the EU has issued Regulations on different categories of medical devices. Medical device manufacturers from
all over the world must follow the Regulations if they want to place their products on the European market.
In case that a manufacturer intends to place its product on the EU market, the product shall be CE marked,
which is a prerequisite for obtaining the qualification to export medical devices to the EU market.
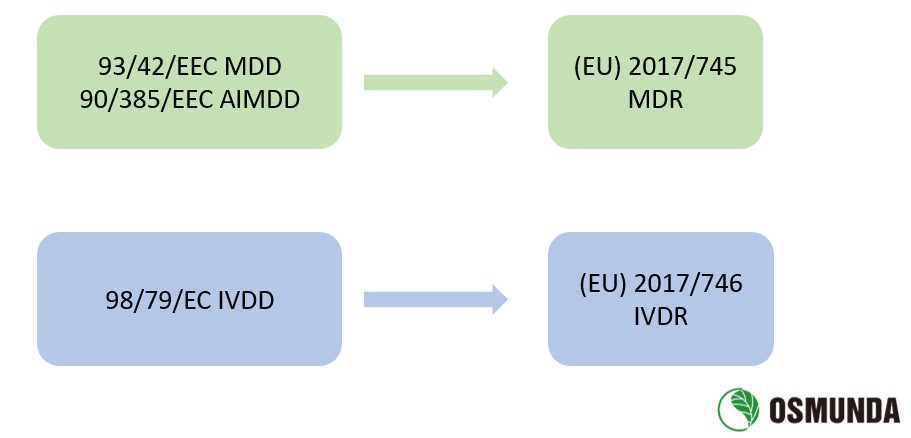
The EU issued three Directives on medical devices in the 1990s:
1) Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating
to active implantable medical devices (AIMDD, 90/385/EEC);
2) Council Directive 93/42/EEC of 14 June 1993 concerning medical devices (MDD, 93/42/EEC);
3) Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic
medical devices (IVDD, 98/79/EC)
On 5 May 2017, the EU published Regulation (EU) 2017/745 of the European Parliament and of the Council on
medical devices (MDR, enter into force on 26 May 2017) and Regulation (EU) 2017/746 of the European Parliament
and of the Council on in vitro diagnostic medical devices (IVDR, enter into force on 26 May 2017) in the Official
Journal of the European Union. MDR replaced MDD and AIMDD, while IVDR replaced IVDD.
According to MDR, medical devices are classified into four different classes: Class I, Class IIa, Class IIb and Class III,
with the risk from low to high. Similarly, IVD devices are classified into Class A, Class B, Class C and Class D based on
IVDR. Regardless of the class, all products should be registered with the authorities before being placed on the EU
market.
Osmunda’s global team could act as your European Authorized Representative, help you with the technical file
preparation, apply and communicate with the authorities and the notified body (NB), and provide clinical trial services.
PRCC
According to European Regulations on Medical Devices and in vitro diagnostic medical devices, manufacturers shall
have available at least one person responsible for regulatory compliance who possess demonstrated expertise in the
field of medical devices.
The Person Responsible for Regulatory Compliance (PRRC) shall be at least responsible for ensuring that:
a)Conformity of devices are appropriated with the relevant Quality Management System (QMS)
b)The technical documentation and EU DoC are up-to date
c)Post market surveillance obligations are properly performed
d)Reporting obligation on serious incidents and field safety corrective actions are met
Manufacturers would be wise to hire this position with qualified professionals to:
1.Be compliant with the EU regulatory requirements
2.Ensure the safety and compliance of the marketed devices
3.Avoid any financial penalties or reputational harm
If you need a PRRC, Contact us to know what Osmunda can do for you!